What Happens When Gold Reacts With Acid . Some metals, like copper, gold and silver do not react with. metals above hydrogen in the reactivity series react with acids; Au (s) + hno 3 (aq) gold dissolves in aqua regia, a. reaction of gold with acids. Naming the salt from the reaction of a metal and an acid. gold reacts with acids to produce lots of bubbles. metals such as lead, tin, magnesium and iron react with dilute acids. Those below hydrogen in the reactivity series don't. gold, for example, does not even react with nitric acid, a strong oxidizing agent, although it will dissolve in aqua. when metals react with acid, we see bubbles forming, so we know chemical reaction has taken place. Gold does not dissolve in nitric acid, hno 3 [4]. However, it can be dissolved. gold does not react with most acids, including hydrochloric acid and sulfuric acid.
from www.mdpi.com
Naming the salt from the reaction of a metal and an acid. reaction of gold with acids. gold reacts with acids to produce lots of bubbles. metals above hydrogen in the reactivity series react with acids; Gold does not dissolve in nitric acid, hno 3 [4]. Au (s) + hno 3 (aq) gold dissolves in aqua regia, a. Those below hydrogen in the reactivity series don't. Some metals, like copper, gold and silver do not react with. gold, for example, does not even react with nitric acid, a strong oxidizing agent, although it will dissolve in aqua. gold does not react with most acids, including hydrochloric acid and sulfuric acid.
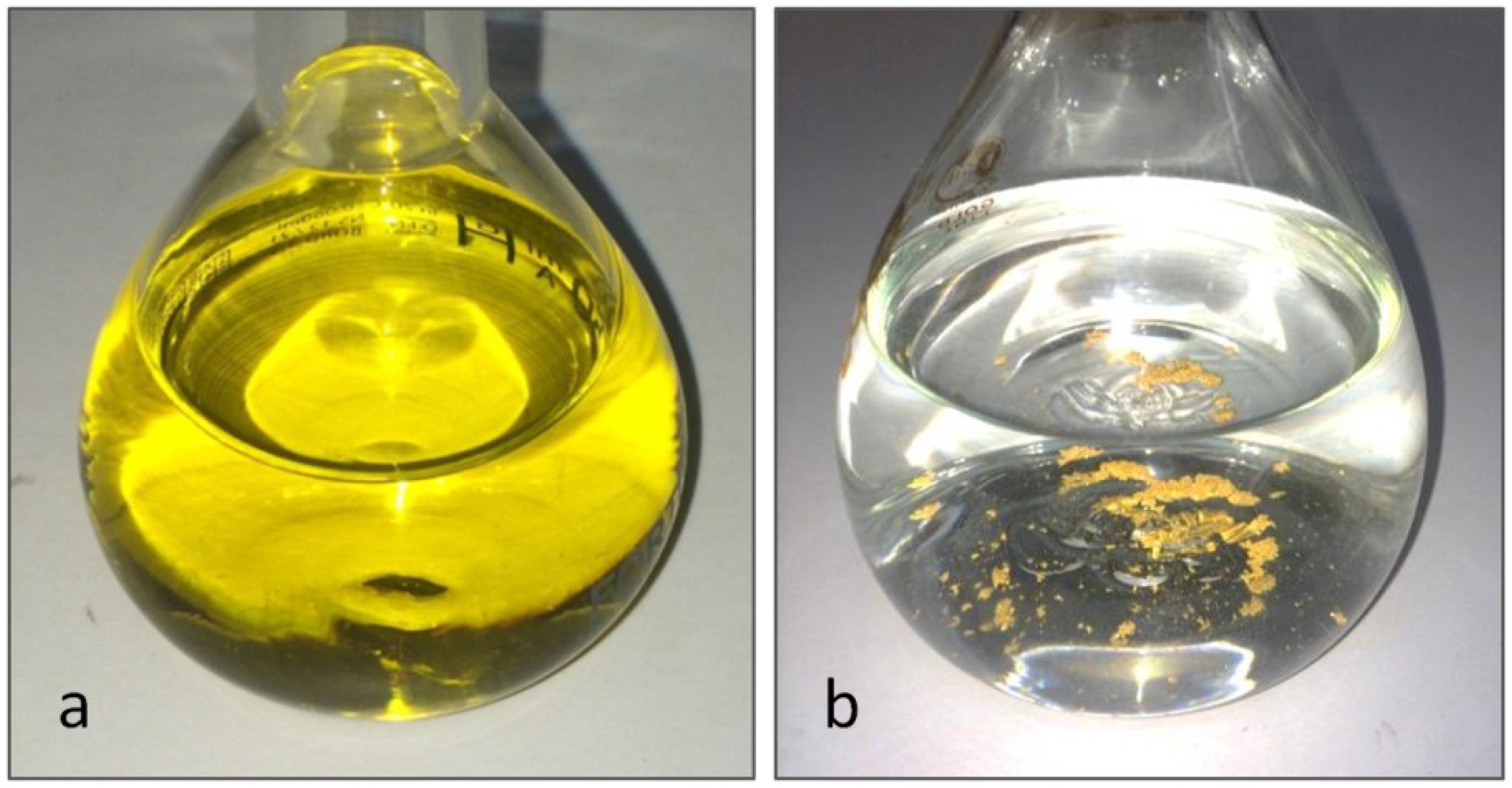
Metals Free FullText A Straightforward Route to Tetrachloroauric
What Happens When Gold Reacts With Acid Au (s) + hno 3 (aq) gold dissolves in aqua regia, a. Au (s) + hno 3 (aq) gold dissolves in aqua regia, a. gold reacts with acids to produce lots of bubbles. Gold does not dissolve in nitric acid, hno 3 [4]. Naming the salt from the reaction of a metal and an acid. However, it can be dissolved. Some metals, like copper, gold and silver do not react with. metals above hydrogen in the reactivity series react with acids; reaction of gold with acids. gold does not react with most acids, including hydrochloric acid and sulfuric acid. when metals react with acid, we see bubbles forming, so we know chemical reaction has taken place. Those below hydrogen in the reactivity series don't. gold, for example, does not even react with nitric acid, a strong oxidizing agent, although it will dissolve in aqua. metals such as lead, tin, magnesium and iron react with dilute acids.
From www.youtube.com
Metals reacting with acid Metals and non metals Chemistry Khan What Happens When Gold Reacts With Acid Those below hydrogen in the reactivity series don't. gold does not react with most acids, including hydrochloric acid and sulfuric acid. when metals react with acid, we see bubbles forming, so we know chemical reaction has taken place. Naming the salt from the reaction of a metal and an acid. Some metals, like copper, gold and silver do. What Happens When Gold Reacts With Acid.
From www.chegg.com
Solved Gold reacts with nitric acid and hydrochloric acid What Happens When Gold Reacts With Acid Those below hydrogen in the reactivity series don't. However, it can be dissolved. metals above hydrogen in the reactivity series react with acids; Some metals, like copper, gold and silver do not react with. gold, for example, does not even react with nitric acid, a strong oxidizing agent, although it will dissolve in aqua. when metals react. What Happens When Gold Reacts With Acid.
From www.slideserve.com
PPT Gold PowerPoint Presentation, free download ID2232139 What Happens When Gold Reacts With Acid Au (s) + hno 3 (aq) gold dissolves in aqua regia, a. when metals react with acid, we see bubbles forming, so we know chemical reaction has taken place. gold, for example, does not even react with nitric acid, a strong oxidizing agent, although it will dissolve in aqua. metals above hydrogen in the reactivity series react. What Happens When Gold Reacts With Acid.
From www.slideshare.net
REACTION OF METALS WITH ACID What Happens When Gold Reacts With Acid metals such as lead, tin, magnesium and iron react with dilute acids. Naming the salt from the reaction of a metal and an acid. Gold does not dissolve in nitric acid, hno 3 [4]. gold, for example, does not even react with nitric acid, a strong oxidizing agent, although it will dissolve in aqua. gold does not. What Happens When Gold Reacts With Acid.
From www.youtube.com
How To Test Gold with Nitric Acid YouTube What Happens When Gold Reacts With Acid Gold does not dissolve in nitric acid, hno 3 [4]. Naming the salt from the reaction of a metal and an acid. gold, for example, does not even react with nitric acid, a strong oxidizing agent, although it will dissolve in aqua. gold does not react with most acids, including hydrochloric acid and sulfuric acid. gold reacts. What Happens When Gold Reacts With Acid.
From www.youtube.com
Au + HCl (Gold + Hydrochloric acid) Equation YouTube What Happens When Gold Reacts With Acid gold does not react with most acids, including hydrochloric acid and sulfuric acid. Naming the salt from the reaction of a metal and an acid. Gold does not dissolve in nitric acid, hno 3 [4]. Au (s) + hno 3 (aq) gold dissolves in aqua regia, a. However, it can be dissolved. when metals react with acid, we. What Happens When Gold Reacts With Acid.
From www.numerade.com
⏩SOLVEDGold metal will not dissolve in either concentrated nitric What Happens When Gold Reacts With Acid gold, for example, does not even react with nitric acid, a strong oxidizing agent, although it will dissolve in aqua. metals such as lead, tin, magnesium and iron react with dilute acids. Naming the salt from the reaction of a metal and an acid. when metals react with acid, we see bubbles forming, so we know chemical. What Happens When Gold Reacts With Acid.
From www.youtube.com
Aqua regia process dissolving gold recovery reaction equation YouTube What Happens When Gold Reacts With Acid Naming the salt from the reaction of a metal and an acid. gold, for example, does not even react with nitric acid, a strong oxidizing agent, although it will dissolve in aqua. when metals react with acid, we see bubbles forming, so we know chemical reaction has taken place. Those below hydrogen in the reactivity series don't. . What Happens When Gold Reacts With Acid.
From www.mdpi.com
Analytica Free FullText Gold Nanoparticles A Didactic Stepby What Happens When Gold Reacts With Acid gold reacts with acids to produce lots of bubbles. Those below hydrogen in the reactivity series don't. Au (s) + hno 3 (aq) gold dissolves in aqua regia, a. Naming the salt from the reaction of a metal and an acid. metals above hydrogen in the reactivity series react with acids; reaction of gold with acids. . What Happens When Gold Reacts With Acid.
From www.youtube.com
Make Gold from Chloroauric Acid YouTube What Happens When Gold Reacts With Acid Those below hydrogen in the reactivity series don't. gold reacts with acids to produce lots of bubbles. However, it can be dissolved. reaction of gold with acids. metals such as lead, tin, magnesium and iron react with dilute acids. Gold does not dissolve in nitric acid, hno 3 [4]. Au (s) + hno 3 (aq) gold dissolves. What Happens When Gold Reacts With Acid.
From www.trymintly.com
How to tell if Gold is real with simple gold tests Mintly What Happens When Gold Reacts With Acid Some metals, like copper, gold and silver do not react with. metals above hydrogen in the reactivity series react with acids; gold reacts with acids to produce lots of bubbles. gold, for example, does not even react with nitric acid, a strong oxidizing agent, although it will dissolve in aqua. Those below hydrogen in the reactivity series. What Happens When Gold Reacts With Acid.
From www.mdpi.com
Metals Free FullText A Straightforward Route to Tetrachloroauric What Happens When Gold Reacts With Acid Those below hydrogen in the reactivity series don't. gold does not react with most acids, including hydrochloric acid and sulfuric acid. metals above hydrogen in the reactivity series react with acids; Au (s) + hno 3 (aq) gold dissolves in aqua regia, a. Some metals, like copper, gold and silver do not react with. Naming the salt from. What Happens When Gold Reacts With Acid.
From www.youtube.com
What happens when metals react with acid ? Ch. 3 Class10 What Happens When Gold Reacts With Acid metals such as lead, tin, magnesium and iron react with dilute acids. Some metals, like copper, gold and silver do not react with. reaction of gold with acids. Naming the salt from the reaction of a metal and an acid. However, it can be dissolved. gold does not react with most acids, including hydrochloric acid and sulfuric. What Happens When Gold Reacts With Acid.
From www.researchgate.net
Ascorbic acid reduction mechanism of gold and silver ions to obtain Ag What Happens When Gold Reacts With Acid gold reacts with acids to produce lots of bubbles. Some metals, like copper, gold and silver do not react with. gold does not react with most acids, including hydrochloric acid and sulfuric acid. Those below hydrogen in the reactivity series don't. Gold does not dissolve in nitric acid, hno 3 [4]. However, it can be dissolved. reaction. What Happens When Gold Reacts With Acid.
From pubs.acs.org
Capture and Extraction of Gold from Aqueous Solution via the Iron What Happens When Gold Reacts With Acid Au (s) + hno 3 (aq) gold dissolves in aqua regia, a. gold, for example, does not even react with nitric acid, a strong oxidizing agent, although it will dissolve in aqua. gold does not react with most acids, including hydrochloric acid and sulfuric acid. metals such as lead, tin, magnesium and iron react with dilute acids.. What Happens When Gold Reacts With Acid.
From readthescience.blogspot.com
READ THE SCIENCE 10.3The reaction of metals with acids What Happens When Gold Reacts With Acid Naming the salt from the reaction of a metal and an acid. Those below hydrogen in the reactivity series don't. metals above hydrogen in the reactivity series react with acids; gold does not react with most acids, including hydrochloric acid and sulfuric acid. gold reacts with acids to produce lots of bubbles. gold, for example, does. What Happens When Gold Reacts With Acid.
From www.yaclass.in
How do metal carbonates and metal hydrogencarbonates react with acids What Happens When Gold Reacts With Acid Naming the salt from the reaction of a metal and an acid. However, it can be dissolved. Those below hydrogen in the reactivity series don't. Gold does not dissolve in nitric acid, hno 3 [4]. metals such as lead, tin, magnesium and iron react with dilute acids. reaction of gold with acids. gold reacts with acids to. What Happens When Gold Reacts With Acid.
From fphoto.photoshelter.com
science chemistry redox reaction zinc gold hydrochloric acid What Happens When Gold Reacts With Acid Naming the salt from the reaction of a metal and an acid. Au (s) + hno 3 (aq) gold dissolves in aqua regia, a. gold does not react with most acids, including hydrochloric acid and sulfuric acid. Those below hydrogen in the reactivity series don't. when metals react with acid, we see bubbles forming, so we know chemical. What Happens When Gold Reacts With Acid.